| ISOTHERMIC PROCESS | |
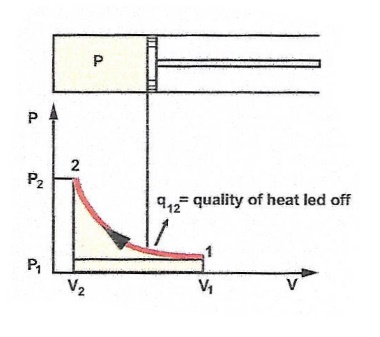
| Isothermic change of state means the temperature of
a gas mixture is constant, when the pressure and volume are changed. |
|
|
q = m x R x T x ln(P2/P1)
q = P1 x V1 x T x ln(V2/V1) |
 |
|
q : The quantity of heat ( j )
m : mass ( Kg ) V : volume (m3) T : absolute temperature ( K ) R : individual gas constant (J/Kg x K) P : absolute pressure ( Pa ) |
|